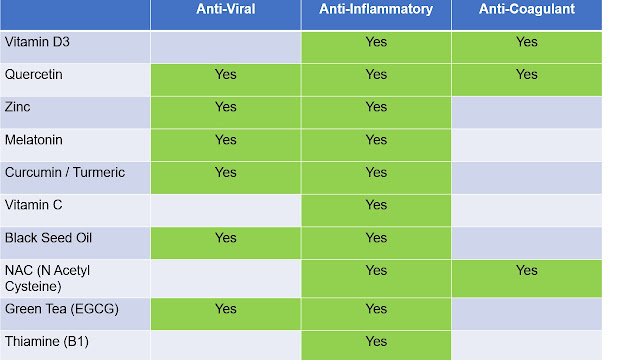
Stem cell treatment for paralyzed patients offers improved chance of independent life
A spinal cord injury is devastating, changing a person’s life in a heartbeat. In the past there was little that doctors could do other than offer pain relief and physical therapy to try and regain as much muscle function as possible. That’s why the latest results from the CIRM-supported Asterias Biotherapeutics spinal cord injury trial are so encouraging.
Asterias is transplanting what they call AST-OPC1 cells into patients who have suffered injuries that left them paralyzed from the neck down. AST-OPC1 are oligodendrocyte progenitor cells, which develop into cells that support and protect nerve cells in the central nervous system, the area damaged in spinal cord injury. It’s hoped the treatment will restore connections at the injury site, allowing patients to regain some movement and feeling.
The latest results seem to suggest they are doing just that.
In a news release, Asterias reports that of the 25 patients treated in this clinical trial none have experienced serious side effects. They also reported that magnetic resonance imaging (MRI) tests show that more than 95 percent of the patients have shown evidence of what’s called “tissue matrix” at the injury site. This is encouraging because it suggests the implanted cells are engrafting and helping prevent a cavitation, a serious process that often occurs in spinal cord injuries and can lead to permanent loss of muscle and sensory function plus chronic pain.
 |
| Kris Boesen, CIRM spinal cord injury clinical trial patient works to strengthen his upper body. (Photo/Greg Iger) |
Asterias is transplanting what they call AST-OPC1 cells into patients who have suffered injuries that left them paralyzed from the neck down. AST-OPC1 are oligodendrocyte progenitor cells, which develop into cells that support and protect nerve cells in the central nervous system, the area damaged in spinal cord injury. It’s hoped the treatment will restore connections at the injury site, allowing patients to regain some movement and feeling.
The latest results seem to suggest they are doing just that.
In a news release, Asterias reports that of the 25 patients treated in this clinical trial none have experienced serious side effects. They also reported that magnetic resonance imaging (MRI) tests show that more than 95 percent of the patients have shown evidence of what’s called “tissue matrix” at the injury site. This is encouraging because it suggests the implanted cells are engrafting and helping prevent a cavitation, a serious process that often occurs in spinal cord injuries and can lead to permanent loss of muscle and sensory function plus chronic pain.
The study also shows that after six months:
“The results from the study remain encouraging as the six-month follow-up data continued to demonstrate a positive safety profile and show that the AST-OPC1 cells are successfully engrafting in patients.”
While none of the patients are able to walk, just regaining some use of their arms or hands can have a hugely important impact on their quality of life and their ability to lead an independent life. And, because lifetime costs of taking care of someone who is paralyzed from the neck or chest down can run as high as $5 million, anything that increases a patient’s independence can have a big impact on those costs.
The impact of this research is helping change the lives of the patients who received it. One of those patients is Jake Javier. We have blogged about Jake several times over the last two years and recently showed this video about his first year at Cal Poly and how Jake is turning what could have been a life-ending event into a life-affirming one.
- 100 percent of the patients in Group 5 (who received 20 million cells) have recovered at least one motor level (for example increased ability to use their arms) on at least one side
- Two patients in Group 5 recovered one motor level on both sides
- Altogether four of the 25 patients have recovered two or more motor levels on at least one side.
“The results from the study remain encouraging as the six-month follow-up data continued to demonstrate a positive safety profile and show that the AST-OPC1 cells are successfully engrafting in patients.”
While none of the patients are able to walk, just regaining some use of their arms or hands can have a hugely important impact on their quality of life and their ability to lead an independent life. And, because lifetime costs of taking care of someone who is paralyzed from the neck or chest down can run as high as $5 million, anything that increases a patient’s independence can have a big impact on those costs.
The impact of this research is helping change the lives of the patients who received it. One of those patients is Jake Javier. We have blogged about Jake several times over the last two years and recently showed this video about his first year at Cal Poly and how Jake is turning what could have been a life-ending event into a life-affirming one.
Category: Stem Cell
.png)
.png)

.jpg)

.png)


.png)
Comments